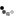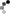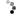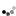bonjour,
je suis étudiante en M1.
Voici un article tiré d'une revue et je n'arrive pas bien à comprendre le but de l'expérience (attenton c'est en anglais mais facile à déchiffrer !)
merci de m'aider
Enfait je ne comprend pas la notion de turn over overall ! et pourquoi on mesure avec un quench-flow, ni pourquoi on obtient de telles courbes.
P.S. : j'ai mis la figure en annexe
Homocitrulline Peptide Displays Rapid Nicotinamide Formation but Slow Overall Turnover Rates—To determine the individual rate constants in the mechanism of ADPr formation with homocitrulline peptide, the rate of nicotinamide formation was measured using a rapid-quench flow apparatus. These single turnover reactions yielded a first-order nicotinamide formation rate of 1.9 ± 0.4 s-1 for homocitrulline peptide (Fig. 7A) compared with 6.7 ± 0.9 s-1 for acetyl-lysine peptide (29). At saturating homocitrulline peptide and NAD+ concentrations, the turnover rate (kcat) was (1.2 ± 0.4) x 10-2 s-1 for homocitrulline peptide compared with 0.2 s-1 for acetyl-lysine peptide (29).
FIGURE 7.
A, rapid-quench flow of homocitrulline peptide. Single turnover reactions were done with 325 µM [14C]NAD+, 10 -20 µM homocitrulline peptide, 40 µM Hst2, and 1 mM DTT in 50 mM Tris, pH 7.5 at 25 °C. Time points from 28.5 to 4000 ms were performed using a Hi-Tech RQF-63 rapid quench flow system and analyzed as described under "Experimental Procedures." B, nicotinamide exchange reaction at varying concentrations of nicotinamide and saturating concentrations of acetyl-lysine (squares) or homocitrulline (circles) peptide. Exchange reactions contained 500 µM NAD+, 300 µM acetyl-lysine analog peptide, 1 mM DTT, 1 µM Hst2, and 25-1600 µM [14C]nicotinamide in 50 mM Tris, pH 7.5 at 25 °C. Exchange rates were determined as outlined under "Experimental Procedures." Error bars represent standard deviations. Points for acetyl-lysine have been published previously (29).
-----